Neural science
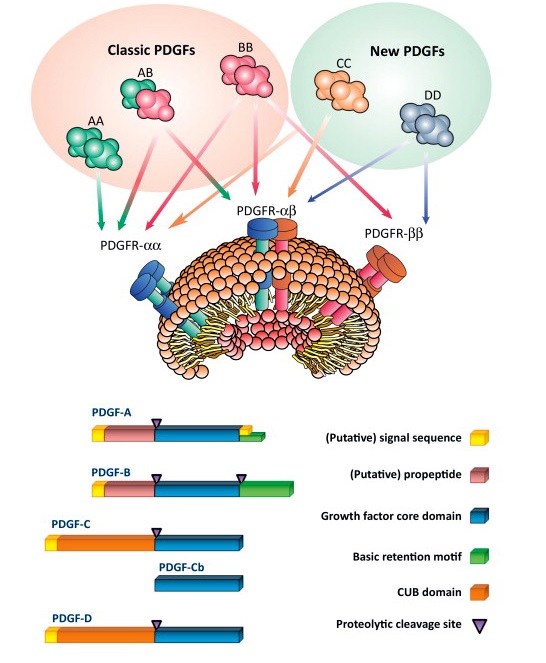
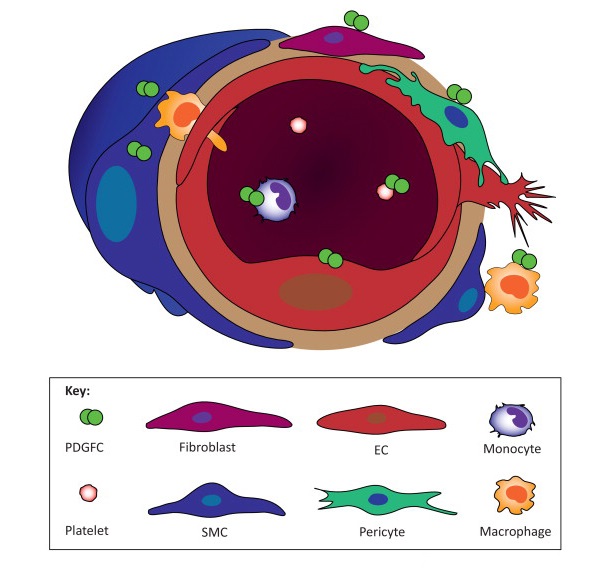
PDGF-C was unexpectedly identified in an attempt to find unknown VEGF homologs. Many studies have reported that PDGF-C plays a critical role in a variety of biological processes since its discovery in 2000 (Li X. et al., Nat Cell Biol., 2000; Li X. et al., Cytokine Growth Factor Rev., 2003; Li X. et al., Oncotarget, 2010; Lee C. et al., Trends Mol Med., 2013). PDGF-C is produced as a latent protein and requires proteolytic processing for receptor binding and activation. PDGF-C binds to the PDGFR-α homodimers and the PDGFR-αβ heterodimers. It is abundantly expressed in many cell types including endothelial cells, vascular smooth muscle cells, pericytes, and fibroblasts (Li X. et al., Cytokine Growth Factor Rev. 2003; Li X. et al., Oncotarget, 2010; Lee C. et al., Trends Mol Med., 2013). During the embryonic stage, PDGF-C is critically regulated for palate formation because PDGF-C deficient mice bred on a 129 background die postnatally.
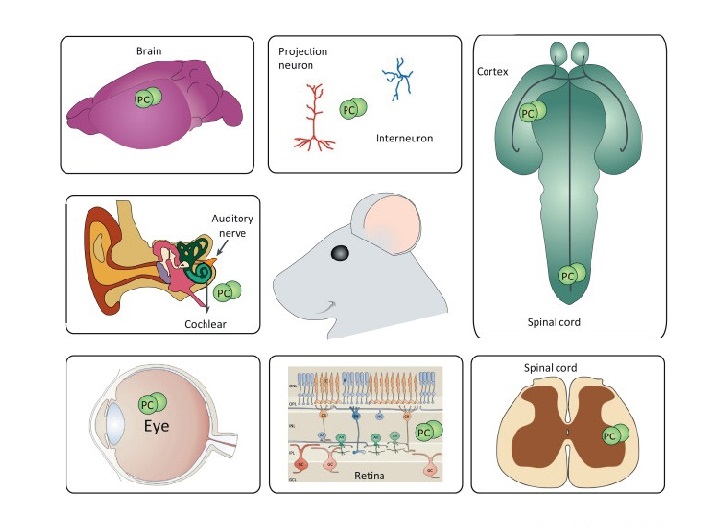
We have recently shown that PDGF-C has neuroprotective effects for neuronal cells by modulating glycogen synthase kinase-3β (GSK-3β) activity (Tang Z. et al., J Exp Med., 2010). In addition to neuroprotective roles of PDGF-C, we have also demonstrated that it is a potent vascular protective and survival factor mediated by heme oxygenase-1 (HMOX-1) (He C. et al., Proc Natl Acad Sci USA, 2014). In conjunction with vascular and neuroprotective roles of PDGF-C, its inhibition by different approaches such as neutralizing Ab, shRNA, or genetic depletion suppressed both choroidal and retinal neovascularization (Hou X. et al., Proc Natl Acad Sci USA, 2010). Collectively, our data show that PDGF-C plays a crucial role in maintenance of blood vessel and neuronal cell survival and its inhibition may be of therapeutic target in treating neovascular diseases.
We are currently investigating the basic science of PDGF-C in terms of vascular and neural effects and possible therapeutic potentials of PDGF-C in ocular and neovascular diseases using proteomics and genetic tools.